Biology and
immunology
of malaria

Our research aims to elucidate fundamental aspects of liver infection by Plasmodium, the agent of malaria, with the aim of identifying and validating new drug and vaccine targets.
Plasmodium is transmitted by a mosquito in the form of sporozoites, which infect the host liver for an initial cycle of replication.
Building on our expertise in sporozoite and liver stage biology and our discoveries of host and parasite factors that play a key role during infection, we are developing projects exploring the mechanisms of sporozoite entry, development of liver forms and hypnozoite formation, gene regulation during transmission, and immunity to liver stages of infection.
Keywords : Paludisme – Plasmodium – Sporozoites – Hypnozoa – Vaccine and medicines
The team members
Our research focus on 4 axes
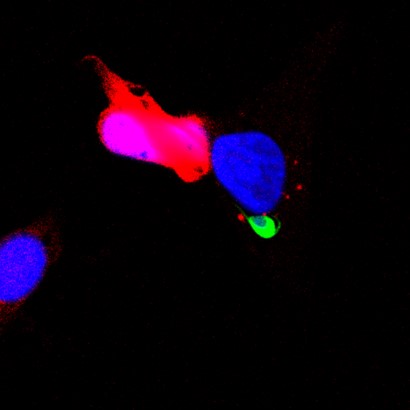
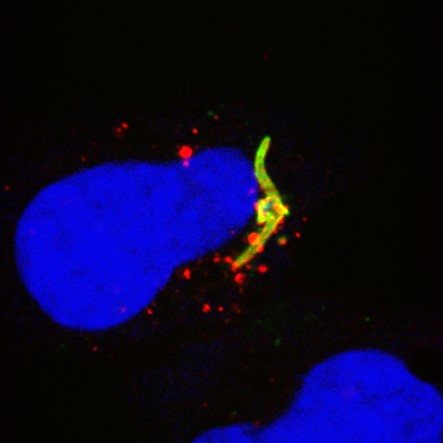
1. Mechanisms of sporozoite entry into hepatocytes
An effective strategy to prevent initial infection at the liver stage is to interfere with the host-parasite molecular interactions that allow sporozoite entry into cells.
Our team has discovered cellular entry pathways and identified key host and parasite proteins involved in the entry process. Our aim is to elucidate the mechanisms of action of these molecular players and to evaluate potential targets for the design of new malaria vaccines.
To this end, we combine experimental genetic approaches and in vitro cellular models, using murine and human parasites.
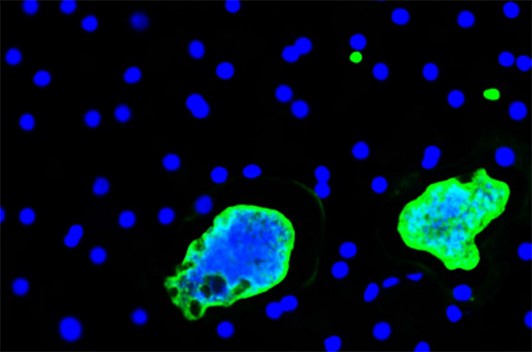
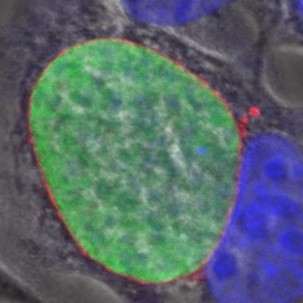
2. Biology of hypnozoites
Some species of Plasmodium such as P. vivax produce dormant forms called hypnozoites, which can reactivate and cause relapses long after transmission by a mosquito. Our group has developed robust cell models for long-term in vitro culture of hypnozoites, and a humanised mouse model for in vivo studies.
These models are used to explore the mechanisms of dormancy and to search for compounds capable of killing hypnozoites or activating them for “wake and kill” strategies.
3. T-cell mediated cellular immunity against hepatic forms of Plasmodium
In mouse models, cytotoxic CD8+ T cells play a major role in protective immunity against hepatic forms of malaria. However, our knowledge of human immunity to the hepatic stages of malaria remains limited.
Furthermore, the nature of the protective antigens is still not defined.
Using transgenic parasites expressing human T-cell epitopes and robust in vitro and in vivo experimental systems set up in the laboratory, including humanised mouse and 3D cell culture models, we are investigating human CD8+ T-cell responses specific to hepatic forms of the parasite and looking for new protective antigenic targets.
Our ultimate goal is to identify correlates of protection, with potential implications for the design of next generation malaria vaccines.
4. Biology of tetraspanins
Tetraspanins are a family of evolutionarily conserved transmembrane proteins that play a key role in many physiological processes.
We discovered the role of CD81 and CD9 in muscle cell fusion during muscle regeneration, and showed that TspanC8 tetraspanins interact directly with the ADAM10 metalloprotease and regulate signalling via the ADAM10 substrate Notch.
Nous avons également contribué à définir le rôle de CD81 lors d’infections par Plasmodium et Listeria. Our work aims to understand the function of these proteins and their ability to organize a dynamic network of interactions at the cell surface.
The opportunities
- New vaccine strategies against malaria
- Screening of active molecules against hepatic forms of Plasmodium
- Antigenic discovery, correlates of protection
Publications
Azithromycin disrupts apicoplast biogenesis in replicating and dormant liver stages of the relapsing malaria parasites Plasmodium vivax and Plasmodium cynomolgi. Int J Antimicrob Agents. 2024 May;63(5):107112. doi: 10.1016/j.ijantimicag.2024.107112. Epub 2024 Feb 15.
Differential proteomics argues against a general role for CD9, CD81 or CD63 in the sorting of proteins into extracellular vesicles. J Extracell Vesicles. 2023 Aug;12(8):e12352. doi: 10.1002/jev2.12352.
The claudin-like apicomplexan microneme protein is required for gliding motility and infectivity of Plasmodium sporozoites. PLoS Pathog. 2023 Mar 16;19(3):e1011261. doi: 10.1371/journal.ppat.1011261. eCollection 2023 Mar.
Plasmodium sporozoites require the protein B9 to invade hepatocytes. iScience. 2023 Jan 25;26(2):106056. doi: 10.1016/j.isci.2023.106056. eCollection 2023 Feb 17.
The AMA1-RON complex drives Plasmodium sporozoite invasion in the mosquito and mammalian hosts. PLoS Pathog. 2022 Jun 22;18(6):e1010643. doi: 10.1371/journal.ppat.1010643. eCollection 2022 Jun.
Artemisinin-independent inhibitory activity of Artemisia sp. infusions against different Plasmodium stages including relapse-causing hypnozoites. Life Sci Alliance. 2021 Dec 2;5(3):e202101237. doi: 10.26508/lsa.202101237. Print 2022 Mar.
Low immunogenicity of malaria pre-erythrocytic stages can be overcome by vaccination. EMBO Mol Med. 2021 Apr 9;13(4):e13390. doi: 10.15252/emmm.202013390. Epub 2021 Mar 11.
Plasmodium P36 determines host cell receptor usage during sporozoite invasion. Elife. 2017 May 16;6:e25903. doi: 10.7554/eLife.25903.
Malaria Sporozoites Traverse Host Cells within Transient Vacuoles. Cell Host Microbe. 2015 Nov 11;18(5):593-603. doi: 10.1016/j.chom.2015.10.006.
Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med. 2014 Mar;20(3):307-12. doi: 10.1038/nm.3461. Epub 2014 Feb 9.
Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat Commun. 2015 Jul 24;6:7690. doi: 10.1038/ncomms8690.
Normal muscle regeneration requires tight control of muscle cell fusion by tetraspanins CD9 and CD81. Nat Commun. 2013;4:1674. doi: 10.1038/ncomms2675.