Immune
microenvironment and
Immunotherapy

The Team “Immune Microenvironment and Immunotherapy” includes scientists and clinicians with strong and complementary expertise in tumor immunology and immunotherapy in patients and mouse pre-clinical models. Team members are also involved in teaching and collaborate with hospitals and pharmaceutical companies at international level.
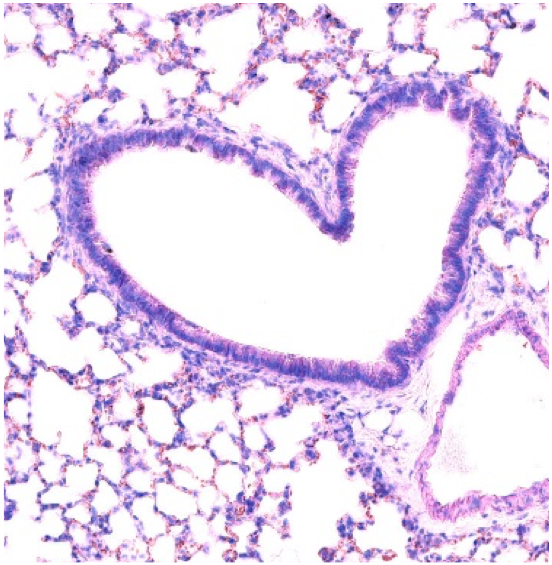
Based on our pioneering work describing the presence and the favorable prognostic value of Tertiary Lymphoid Structures (TLS) in cancer patients, with an in-depth analysis in lung cancer, our core focus is to advance research in the understanding of the cross-talk of TLS with the tumor microenvironment in lung and head/neck cancer patients and their manipulation in pre-clinical models.
Keywords : Tertiary lymphoid structure – Human and murine tumor immunology – T and B cell immunity – Therapeutic manipulation of TLS – Curative vaccination – Lung and head and neck carcinomas
The team members
Our research focus on 3 axes
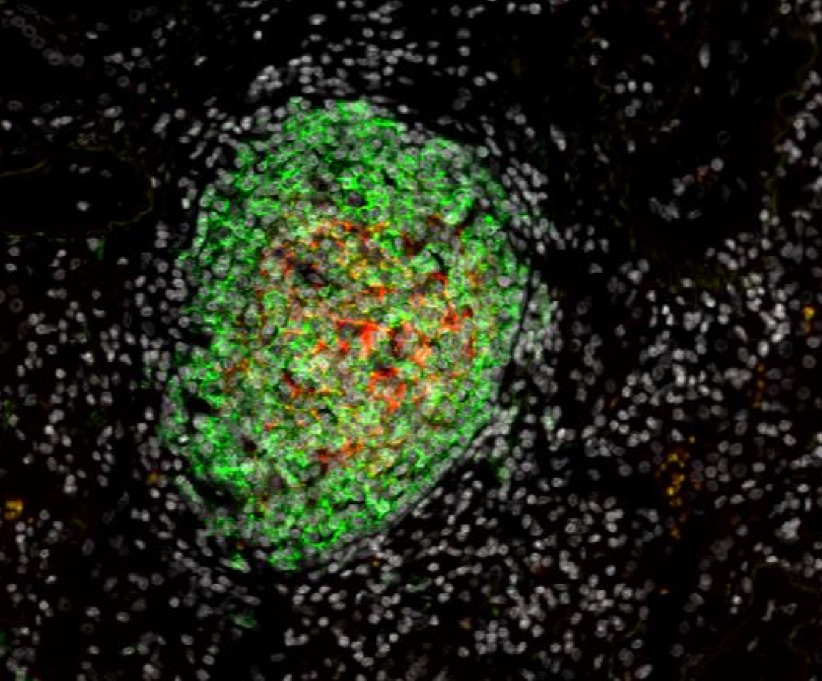
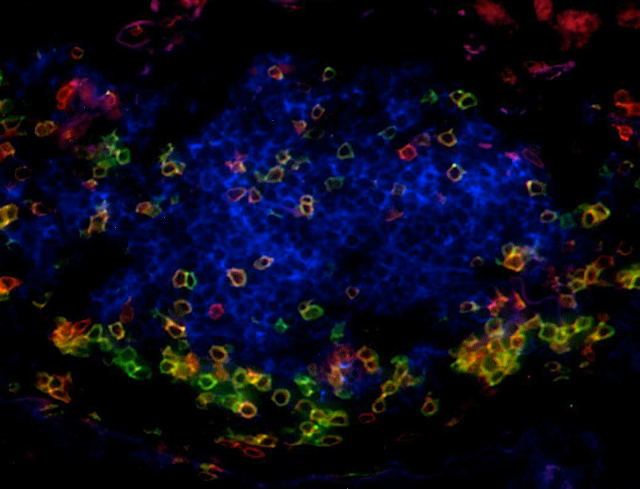
1. Analysis of the immune functions of Tertiary Lymphoid Structures (TLS)
Our work has highlighted that the presence of TLS correlates with T-cell activation, Th1 phenotype, and cytotoxic orientation, and is required to license the prognostic value of tumor-infiltrating CD8+ T cells. Moreover, TLS-B cells correlate with the presence of plasma cells secreting antibodies against tumor antigens.
A specific focus is now to decipher the relationship between TLS, the molecular characteristics of tumor cells, and the functionality of B and T cell subsets in the tumor microenvironment in order to identify critical immune cell cross-talks involved in the development of efficient anti-tumor immunity.
2. Characterization of cells and molecules controlling TLS induction and maintenance
Our studies are aimed at exploring the cellular and molecular determinants that are critical in the induction and maintenance of TLS in the tumor microenvironment (lung and head and neck carcinomas).
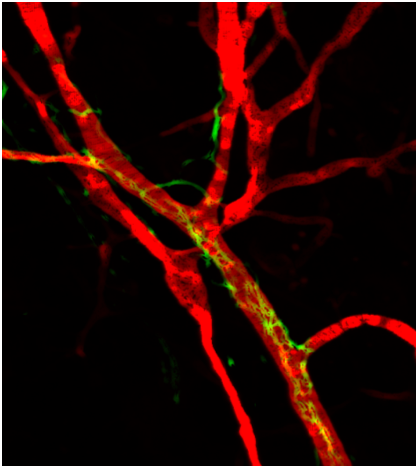
By combining cellular and molecular approaches in mouse models and using human tumor biopsies, our goals are: i) to define the cell population(s) that are involved in the genesis of TLS under various inflammatory conditions; ii) to evaluate the role of neo-vasculature and nerve fibers in the making and maintenance of TLS; iii) to define soluble and membrane molecules that participate to TLS genesis.
3. Development of TLS-based immunotherapies
The presence of TLS in a large number of solid tumors has been associated with a more favorable prognostic.
Thus, the capacity of generating and maintaining functional TLS in the tumor microenvironment (TME) represents an attractive new immunotherapeutic approach to control tumor growth and possibly cure patients by inducing a potent long-lasting immune surveillance against cancer cells.
We are currently developing antibody (Ab)-based fusion molecules and other Ab formats to trigger/reinforce the presence of functional TLS in the TME. Their ability to recruit and stimulate tissue-lymphoid inducer like cells (LTi-like cells) in various models as well as different lipopolyplexe- and virus-based routes of in vivo administration are studied.
The opportunities
- Identification and engineering of molecules that induce TLS neogenesis and stimulate their anti-tumoral role
- Antibody-based combination therapy leading to reshaping the tumor microenvironment
- Optimization of anti-cancer immunotherapy based on new therapeutic vaccines
- Search of new prognostic and predictive biomarkers of cancers
Publications
Absence of sympathetic innervation hampers the generation of tertiary lymphoid structures upon acute lung inflammation. Sci Rep. 2024 May 23;14(1):11749. doi: 10.1038/s41598-024-62673-0.
Enkephalin-mediated modulation of basal somatic sensitivity by regulatory T cells in mice. N Aubert, M Purcarea, M Fornier, L Cagnet, M Naturel, A Casrouge, G Dietrich, M-C Dieu-Nosjean, G Marodon. eLife. 2024 February 1.
[The Nobel Prize in Physiology or Medicine 2023: Katalin Karikó and Drew Weissman – A vaccine revolution driven by fundamental research in immunology and molecular biology]. Med Sci (Paris). 2024 Feb;40(2):186-191. doi: 10.1051/medsci/2024002. Epub 2024 Feb 27.
SAR442085, a novel anti-CD38 antibody with enhanced antitumor activity against multiple myeloma. Blood. 2022 Feb 24;139(8):1160-1176. doi: 10.1182/blood.2021012448. PMID: 35201323
BMFPs, a versatile therapeutic tool for redirecting a preexisting Epstein-Barr virus antibody response toward defined target cells. Sci Adv. 2022 Feb 11;8(6):eabl4363. doi: 10.1126/sciadv.abl4363. Epub 2022 Feb 11. PMID: 35148183 Free PMC article.
Tumor-Associated Tertiary Lymphoid Structures: A Cancer Biomarker and a Target for Next-generation Immunotherapy. Adv Exp Med Biol. 2021;1329:51-68. doi: 10.1007/978-3-030-73119-9_3. PMID: 34664233
Tertiary Lymphoid Structure-B Cells Narrow Regulatory T Cells Impact in Lung Cancer Patients. Front Immunol. 2021 Mar 8;12:626776. doi: 10.3389/fimmu.2021.626776. eCollection 2021. PMID: 33763071 Free PMC article
Blockade of HVEM for Prostate Cancer Immunotherapy in Humanized Mice. Cancers (Basel). 2021 Jun 16;13(12):3009. doi: 10.3390/cancers13123009. Free PMC article.
Tumor-Associated Tertiary Lymphoid Structures: From Basic and Clinical Knowledge to Therapeutic Manipulation. Front Immunol. 2021 Jun 30;12:698604. doi: 10.3389/fimmu.2021.698604. eCollection 2021. PMID: 34276690 Free PMC article.
Effective control of Staphylococcus aureus lung infection despite tertiary lymphoid structure disorganisation. Eur Respir J. 2021 Apr 15;57(4):2000768. doi: 10.1183/13993003.00768-2020. Print 2021 Apr. PMID: 33093122
Intratumoral plasma cells: More than a predictive marker of response to anti-PD-L1 treatment in lung cancer? Cancer Cell. 2022 Mar 14;40(3):240-243. doi: 10.1016/j.ccell.2022.02.008. Epub 2022 Feb 24. PMID: 35216677
A novel combination of chemotherapy and immunotherapy controls tumor growth in mice with a human immune system. Oncoimmunology. 2019 Apr 12;8(7):1596005. . eCollection 2019. Free PMC article.