Chemokines,
Phagocytes
and Inflammation

Our team focus on the origin and function of myeloid cells in infectious diseases and cancer. We try to decipher the regulation of pro and anti-inflammatory activities mediated by these innate immune cells upon insult and understand how the balance tips toward dysfunctional response and worsen the pathology. Our team has developed expertise in multiparametric immuno-monitoring of myeloid cells in patients and mouse preclinical models including flow, mass cytometry and transcriptomic analyses as well as cutting-edge non-linear optical imaging processes to monitor the dynamic of myeloid cells in vivo.
The team members
Our research focus on 3 axes
- Myeloid cells
- Inflammation
- Infection
- Cancer
- Chemokines
- Live imaging
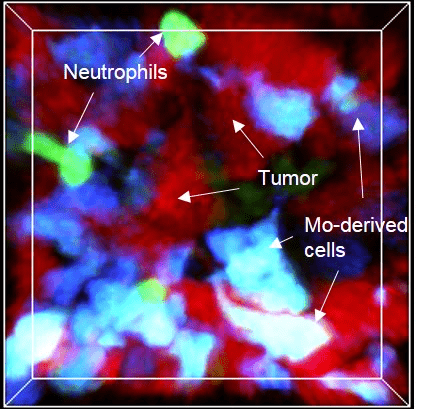
1. Dynamic of the myeloid cells
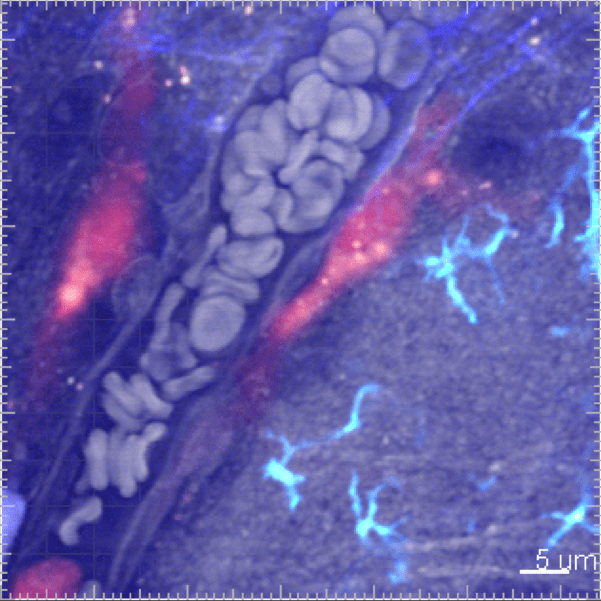
Myeloid cells exert very intense trafficking between the blood vasculature and the tissue with a high turnover. We develop in vivo studies to monitor the kinetic of their mobilization across different tissues and track their behavior by live imaging in animal models.
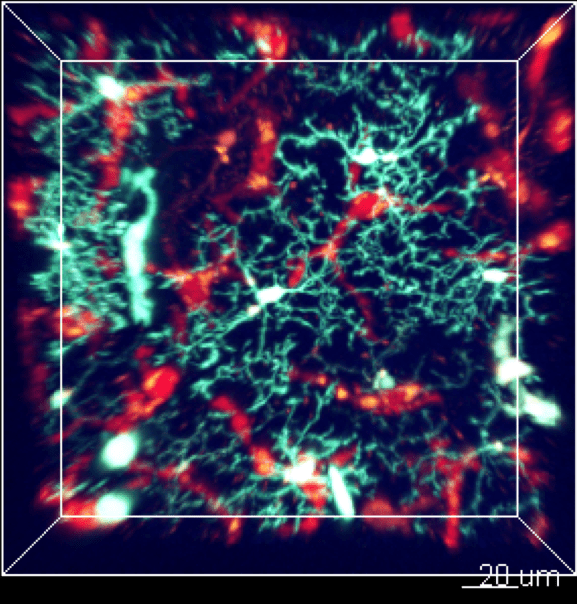
2. Innate immune dysfunction
Innate immune dysfunction represents a major axis in in the pathophysiology of numerous inflammatory diseases such as sepsis, COVID and cancers.
Immunomonitoring the innate immune cells in animal models and human samples through “omic” approaches like mass cytometry, RNA sequencing and multiplex imaging is central in our activity to decipher phenotypic and functional alterations and uncover biomarkers for therapeutic purposes.
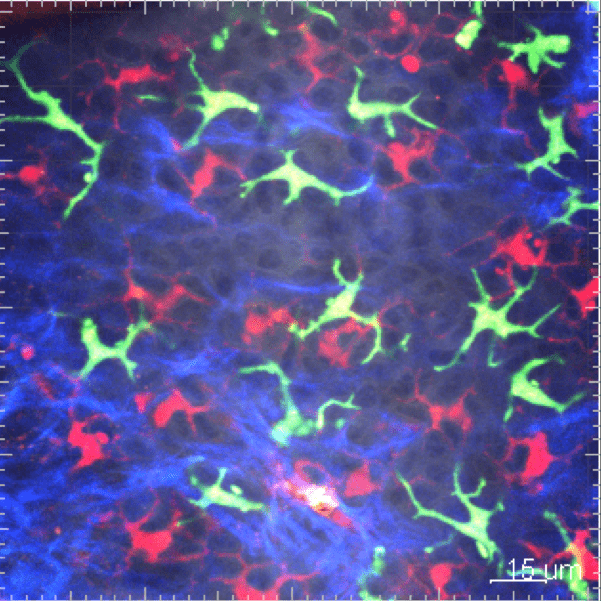
3. Origin and function of phagocytes in inflammation
The field of the mononuclear phagocyte system has significantly evolved this past decade. The concept of phagocyte origin has raised new important questions on the respective contribution of resident and inflammatory macrophages in response to infection, cancer development and tissue injury.
The opportunities
- Technical developments Mass and spectral cytometry, live imaging
- Bench to Bedside (in vivo models and clinical samples)
- Team work
- National and international interactions and collaborations
Publications
Tumor-associated macrophage heterogeneity is driven by tissue territories in breast cancer. Laviron M, Petit M, Weber-Delacroix E, Combes AJ, Arkal AR, Barthélémy S, Courau T, Hume DA, Combadière C, Krummel MF, Boissonnas A. Cell Rep. 2022 May 24;39(8):110865. doi: 10.1016/j.celrep.2022.110865. PMID: 35613577 Free article.
Two New Neutrophil Subsets Define a Discriminating Sepsis Signature. Am J Respir Crit Care Med. 2022 Jan 1;205(1):46-59. doi: 10.1164/rccm.202104-1027OC.
Imaging resident and recruited macrophage contribution to Wallerian degeneration. J Exp Med. 2020 Nov 2;217(11):e20200471. doi: 10.1084/jem.20200471. PMID: 32648893 Free PMC article.
Sepsis Triggers a Late Expansion of Functionally Impaired Tissue-Vascular Inflammatory Monocytes During Clinical Recovery. Front Immunol. 2020 Apr 30;11:675. doi: 10.3389/fimmu.2020.00675. eCollection 2020. PMID: 32425929 Free PMC article.
CCR2-Dependent Recruitment of Tregs and Monocytes Following Radiotherapy Is Associated with TNFα-Mediated Resistance. Cancer Immunol Res. 2019 Mar;7(3):376-387. doi: 10.1158/2326-6066.CIR-18-0633. Epub 2019 Jan 29. PMID: 30696630
Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med. 2018 Oct 1;215(10):2536-2553. doi: 10.1084/jem.20180534. Epub 2018 Sep 10. PMID: 30201786 Free PMC article.
CX3CR1-dependent endothelial margination modulates Ly6Chigh monocyte systemic deployment upon inflammation in mice. Blood. 2017 Mar 9;129(10):1296-1307. doi: 10.1182/blood-2016-08-732164. Epub 2016 Dec 23. PMID: 28011675 Free article.