Signaling and Pathogenesis


The Signaling and Pathogenesis team has been interested in inflammatory and immune processes since its inception, focusing on the NF-kappaB signaling pathway.
Research on the activation and regulation of the immune system is carried out at the molecular and cellular level, but also by using animal models and by studying clinical situations. Our studies on the NF-κB pathway led us to investigate Optineurin, a protein involved in autophagy/mitophagy that shares homologies with NEMO (an essential modulator of the NF-κB pathway).
By discovering new regulators of NF-kappaB and Optineurin, our ambition is to better understand their function and dysregulation in immune, neurodegenerative as well as in cancer.



The team members
Our research focus on 3 axes
- Innate and adaptive immunity
- NF-kappaB signaling
- Autophagy and mitochondria
- SARS-CoV-2
- Neurodegenerative diseases
- Cancer: multiple myeloma and mantle cell lymphoma
1. Identification of a Novel Effector of Antigen-Induced NF-kappaB Signaling
This project demonstrates that the E-Syt2 protein plays an important role in NF-kappaB signaling in response to antigenic stimulation and in the differentiation of iNKT cells, a subset of innate-like T cells that are reactive to glycolipids.
E-Syt2 promotes the formation of junctions between the endoplasmic reticulum and the plasma membrane, enabling lipid exchange between these compartments.
We are working on the hypothesis that E-Syt2 plays a dual role in antitumor immunity by: (1) enabling iNKT cell activation through NF-kappaB signaling, and (2) facilitating the presentation of immunogenic lipids to iNKT cells.
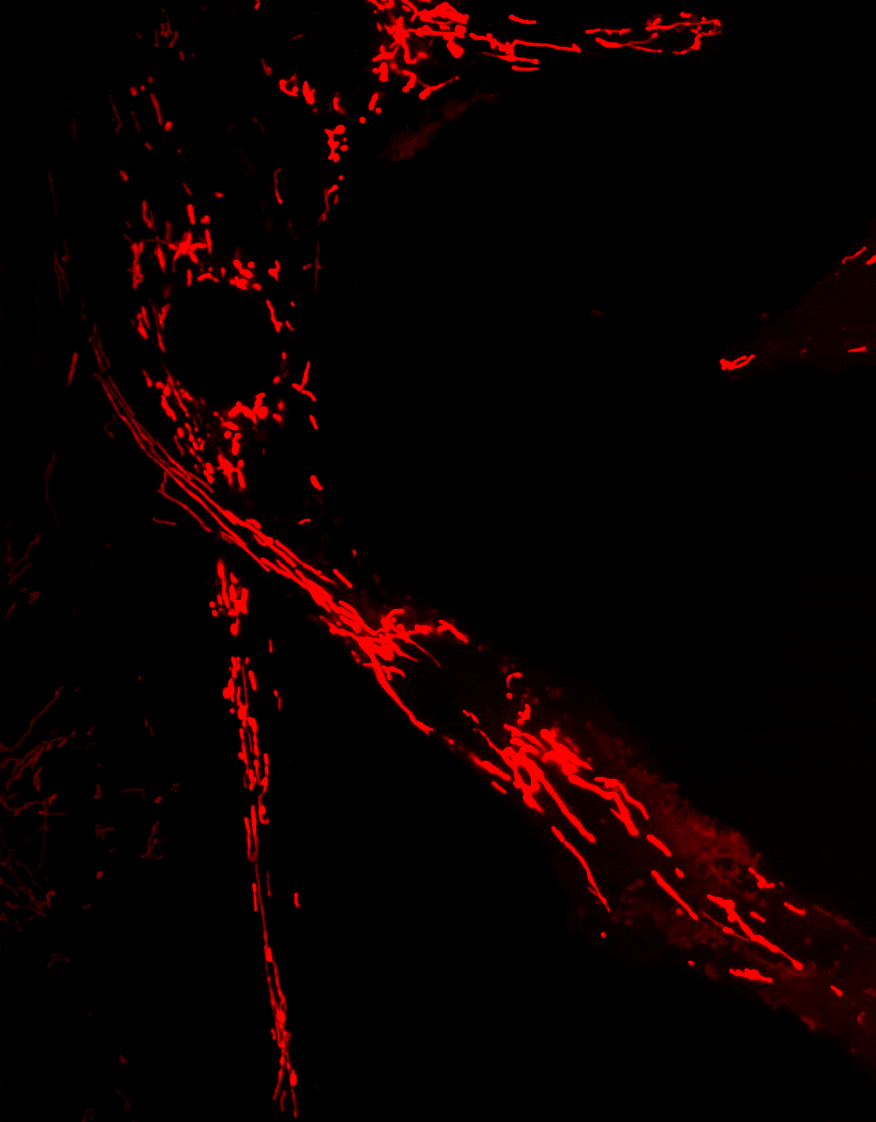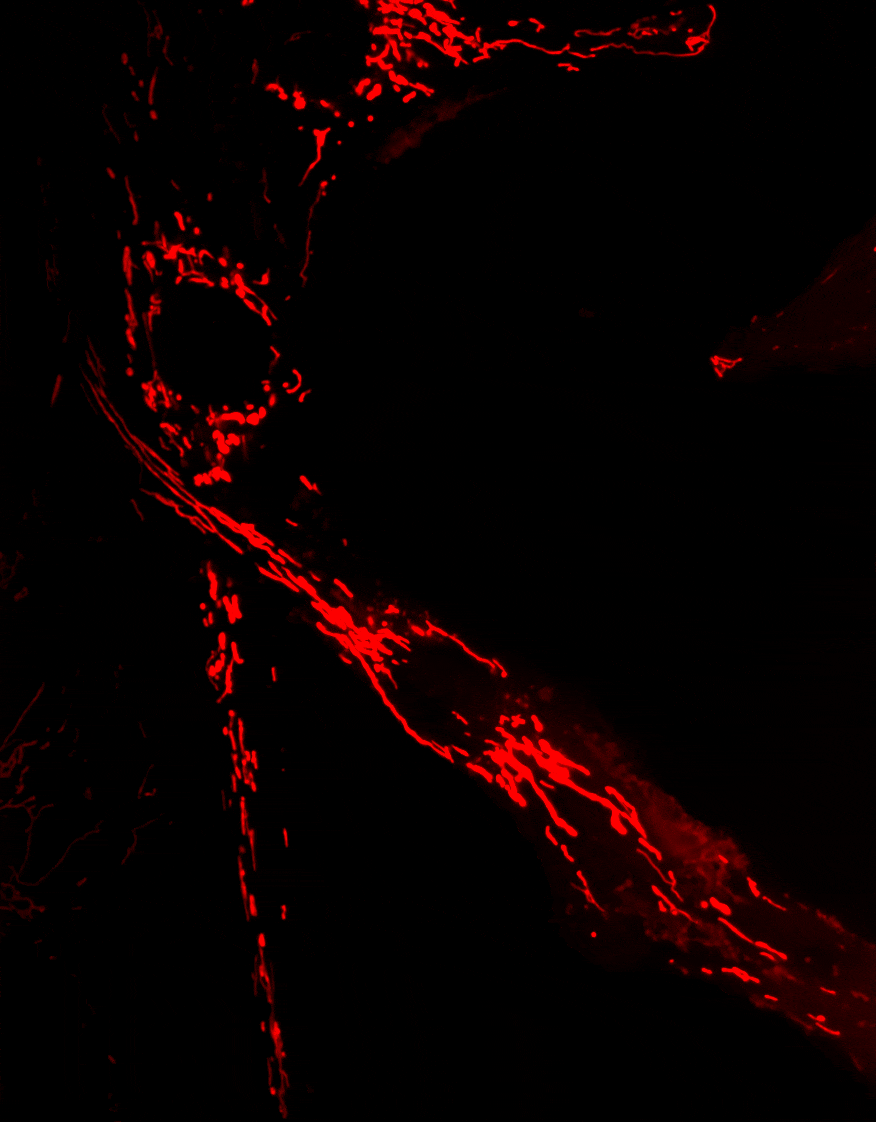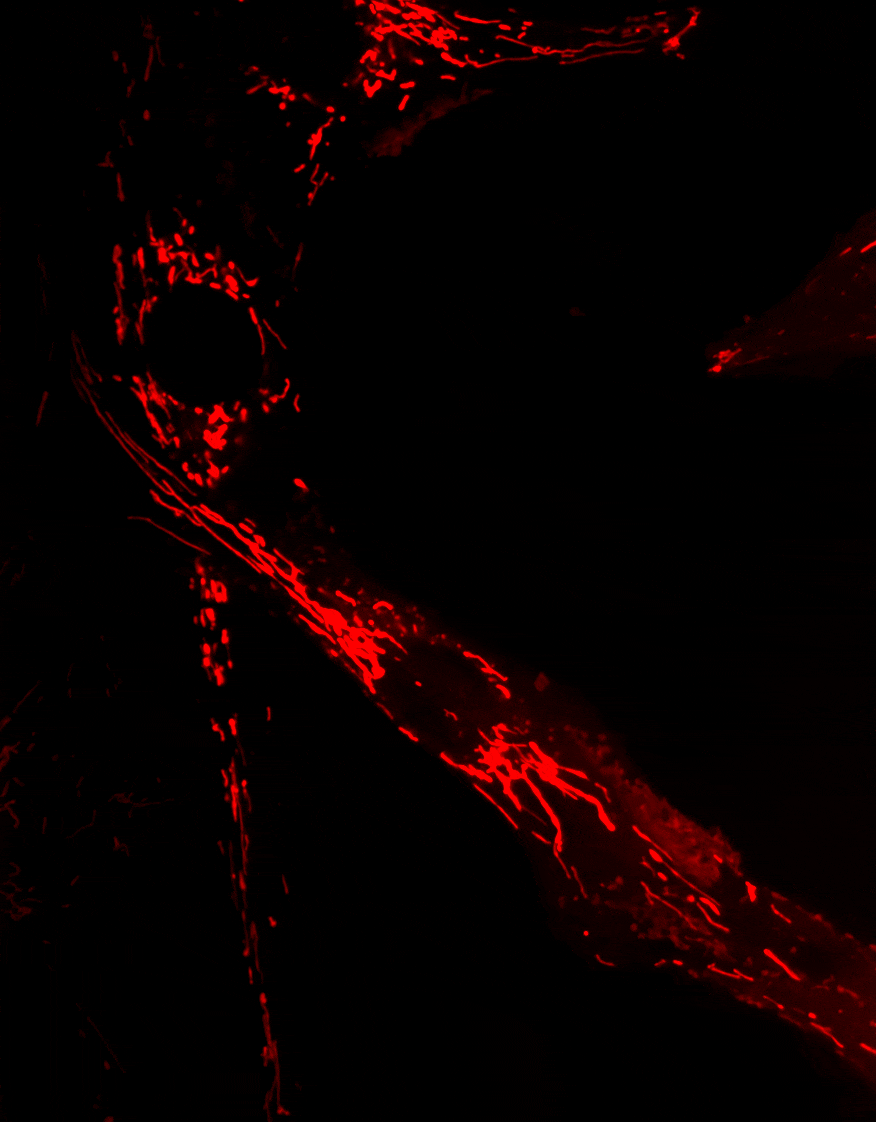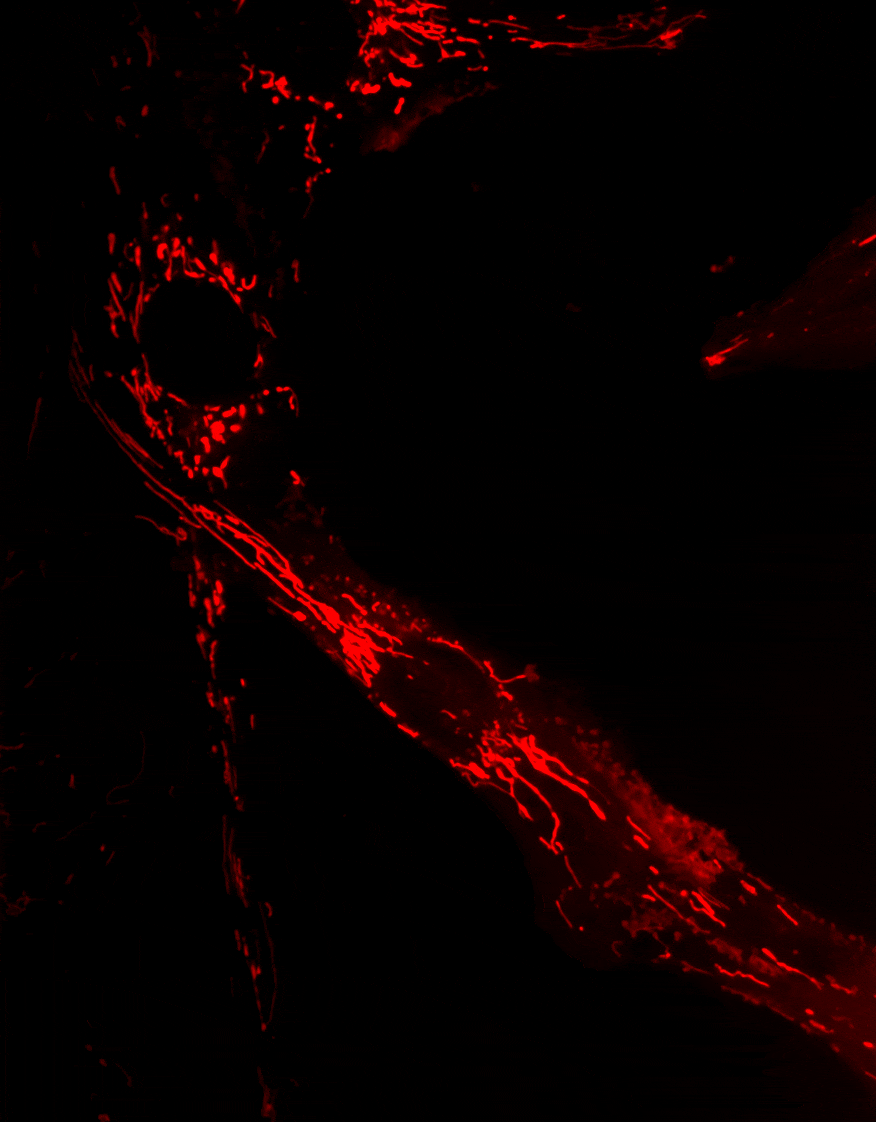
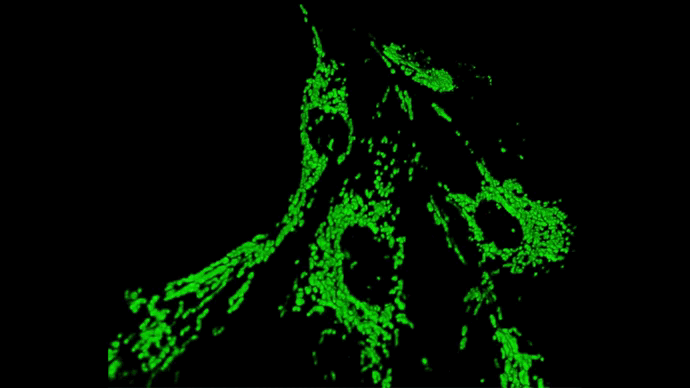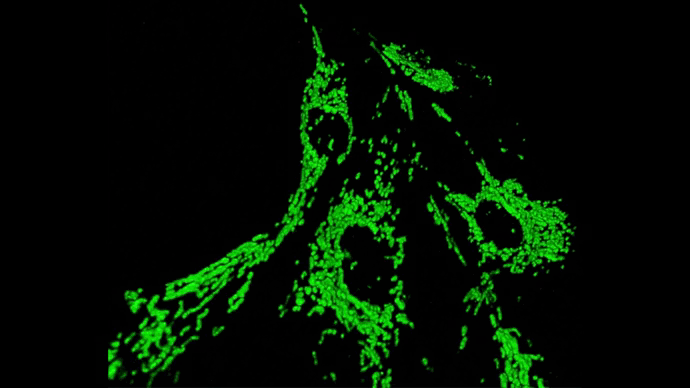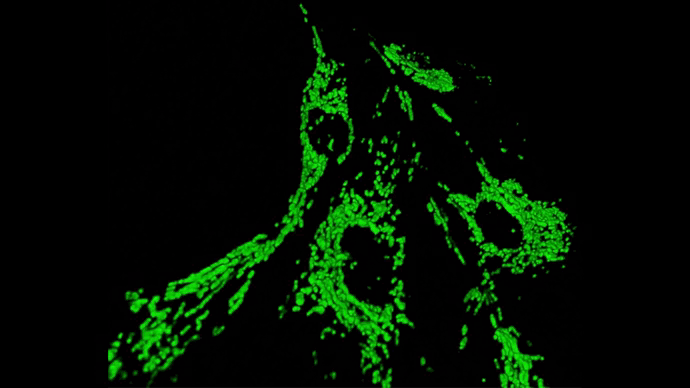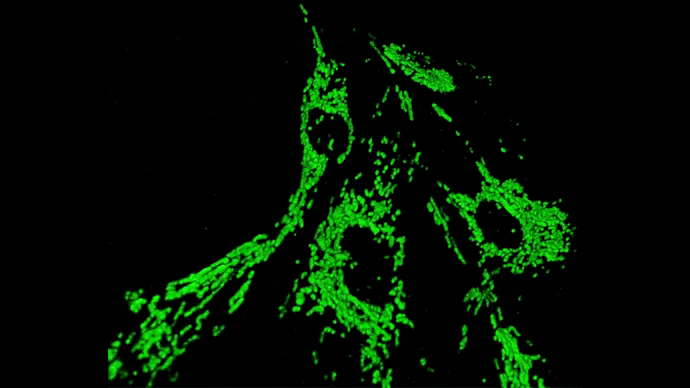
2. Diseases associated with dysfunctions of the autophagy receptor Optineurin
Optineurin (OPTN) is a protein involved in multiple cellular processes, including intracellular membrane trafficking, cell cycle regulation, antiviral immune responses, and selective autophagy.
Mitophagy, the process responsible for the removal of damaged mitochondria, is disrupted in several neurodegenerative diseases. Mutations in the Optn gene have been linked to disorders such as glaucoma and amyotrophic lateral sclerosis (ALS).
We investigate the role of OPTN in mitochondrial transport and clearance, and examine how defects of these biological processes may contribute to the onset and progression of neurodegenerative diseases.
3. NF-κB signaling and the identification of therapeutic targets
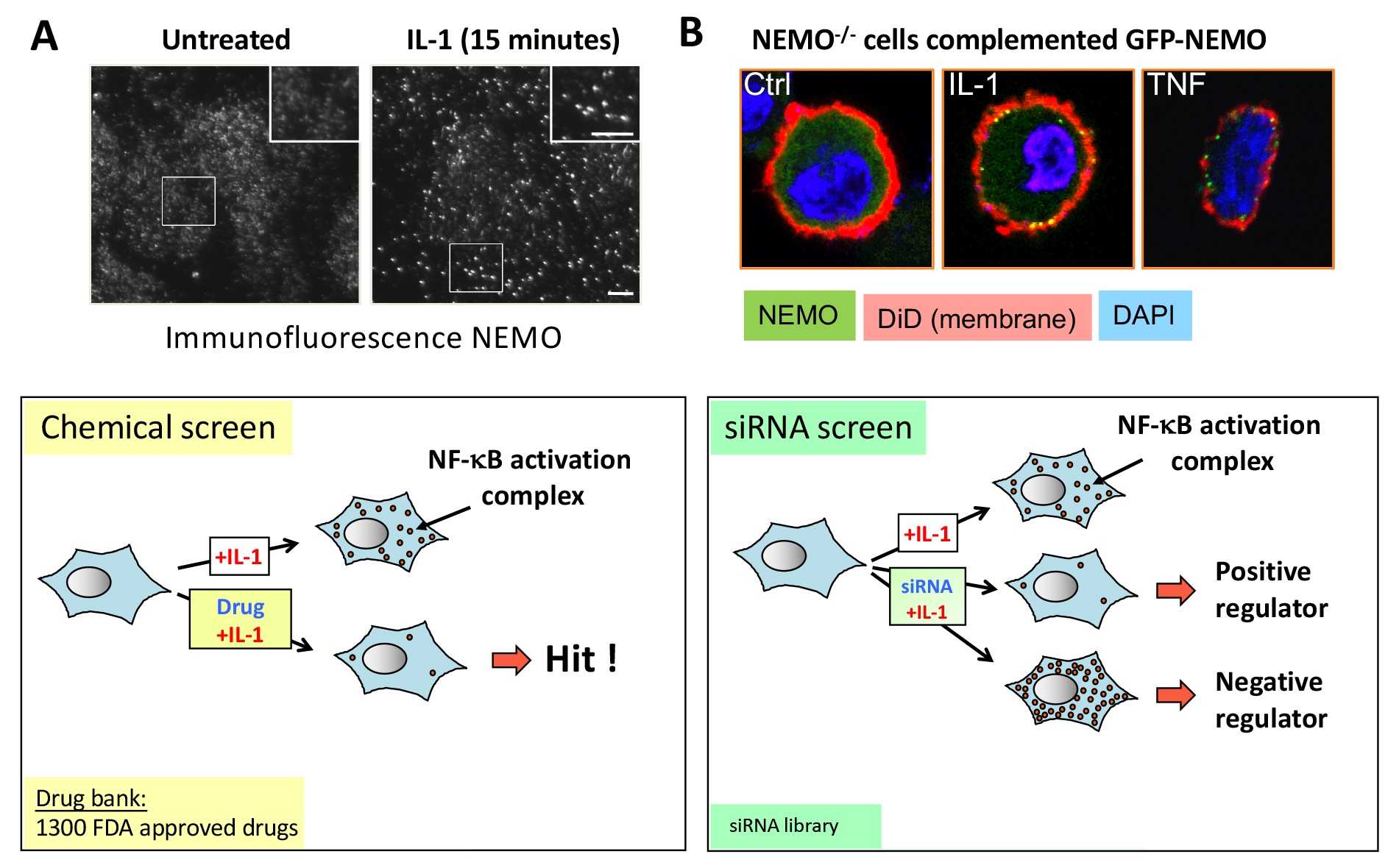
This project investigates the molecular mechanisms regulating the NF-kappaB pathway activated by inflammatory cytokines such as TNF or IL-1, particularly the processes dependent on degradative and non-degradative ubiquitination.
We have shown that IL-1 induces the rapid and transient recruitment of the NEMO/IKK complex to juxta membrane supramolecular structures required for NF-kappaB activation. We used this discovery to perform pharmacological (FDA-approved drugs) and genetic (siRNAs) screens. These approaches led to the identification of novel regulators and inhibitors of these NF-kappaB activation complexes. Notably, we demonstrated that Auranofine inhibits NF-kappaB and blocks the entry of the SARS-CoV-2 virus into cells, making this molecule a potential treatment for COVID-19.
All the identified compounds and regulators are currently undergoing in-depth studies and could constitute new therapeutic targets.
In addition, we study two hematological malignancies, multiple myeloma and mantle cell lymphoma, in which chronic activation of the NF-kappaB pathway contributes to tumor cell survival, with the aim of identifying therapeutic vulnerabilities by targeting this pathway, either alone or in combination with other signaling pathways.
The opportunities
- New therapeutic strategies : immunity / neurodegeneration / cancer
- Drug repurposing for the treatment of COVID-19
- Signaling mechanisms, phosphorylation and ubiquitination
- Cellular compartmentalization and imaging
- Molecular biology, CRISPR–Cas9
Publications
Phosphorylation of Optineurin by protein kinase D regulates Parkin-dependent mitophagy. Weil R, Laplantine E, Attailia M, Oudin A, Curic S, Yokota A, Banide E, Génin P. iScience. 2024 Nov 13;27(12):111384. doi: 10.1016/j.isci.2024.111384. eCollection 2024 Dec 20. PMID: 39669425
The FDA-approved drug Auranofin has a dual inhibitory effect on SARS-CoV-2 entry and NF-κB signaling. iScience. 2022 Sep 3:105066. doi: 10.1016/j.isci.2022.105066. PMID: 36093378 Free PMC article.
NF-κB: At the Borders of Autoimmunity and Inflammation Front Immunol. 2021 Aug 9;12:716469. doi: 10.3389/fimmu.2021.716469. eCollection 2021. PMID: 34434197 Free PMC article.
Two NEMO-like Ubiquitin-Binding Domains in CEP55 Differently Regulate Cytokinesis. Said Halidi KN, Fontan E, Boucharlat A, Davignon L, Charpentier M, Boullé M, Weil R, Israël A, Laplantine E, Agou F. iScience. 2019 Oct 25;20:292-309. doi: 10.1016/j.isci.2019.08.042. Epub 2019 Sep 25. PMID: 31605944
Role of Optineurin in the Mitochondrial Dysfunction: Potential Implications in Neurodegenerative Diseases and Cancer. Front Immunol. 2018 Jun 19;9:1243. doi: 10.3389/fimmu.2018.01243. eCollection 2018. PMID: 29971063 Free PMC article.
Regulation of TBK1 activity by Optineurin contributes to cell cycle-dependent expression of the interferon pathway. Cytokine Growth Factor Rev. 2016 Jun;29:23-33. doi: 10.1016/j.cytogfr.2016.03.001. Epub 2016 Mar 4. PMID: 26976762
Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. Boisson B, Laplantine E, Dobbs K, Cobat A, Tarantino N, Hazen M, Lidov HG, Hopkins G, Du L, Belkadi A, Chrabieh M, Itan Y, Picard C, Fournet JC, Eibel H, Tsitsikov E, Pai SY, Abel L, Al-Herz W, Casanova JL, Israel A, Notarangelo LD. J Exp Med. 2015 Jun 1;212(6):939-51. doi: 10.1084/jem.20141130. Epub 2015 May 25. PMID: 26008899
Optineurin regulates the interferon response in a cell cycle-dependent manner. PLoS Pathog. 2015 Apr 29;11(4):e1004877. doi: 10.1371/journal.ppat.1004877. eCollection 2015 Apr. PMID: 25923723 Free PMC article.
TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO-IKK supramolecular structures. Tarantino N, Tinevez JY, Crowell EF, Boisson B, Henriques R, Mhlanga M, Agou F, Israël A, Laplantine E. J Cell Biol. 2014 Jan 20;204(2):231-45. doi: 10.1083/jcb.201307172. PMID: 24446482
Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Boisson B, Laplantine E, Prando C, Giliani S, Israelsson E, Xu Z, Abhyankar A, Israël L, Trevejo-Nunez G, Bogunovic D, Cepika AM, MacDuff D, Chrabieh M, Hubeau M, Bajolle F, Debré M, Mazzolari E, Vairo D, Agou F, Virgin HW, Bossuyt X, Rambaud C, Facchetti F, Bonnet D, Quartier P, Fournet JC, Pascual V, Chaussabel D, Notarangelo LD, Puel A, Israël A, Casanova JL, Picard C. Nat Immunol. 2012 Dec;13(12):1178-86. doi: 10.1038/ni.2457. Epub 2012 Oct 28. PMID: 23104095
Toward an integrative view of Optineurin functions. Cell Cycle. 2012 Aug 1;11(15):2808-18. doi: 10.4161/cc.20946. Epub 2012 Aug 1. PMID: 22801549