Severe
Malaria
Pathogenesis

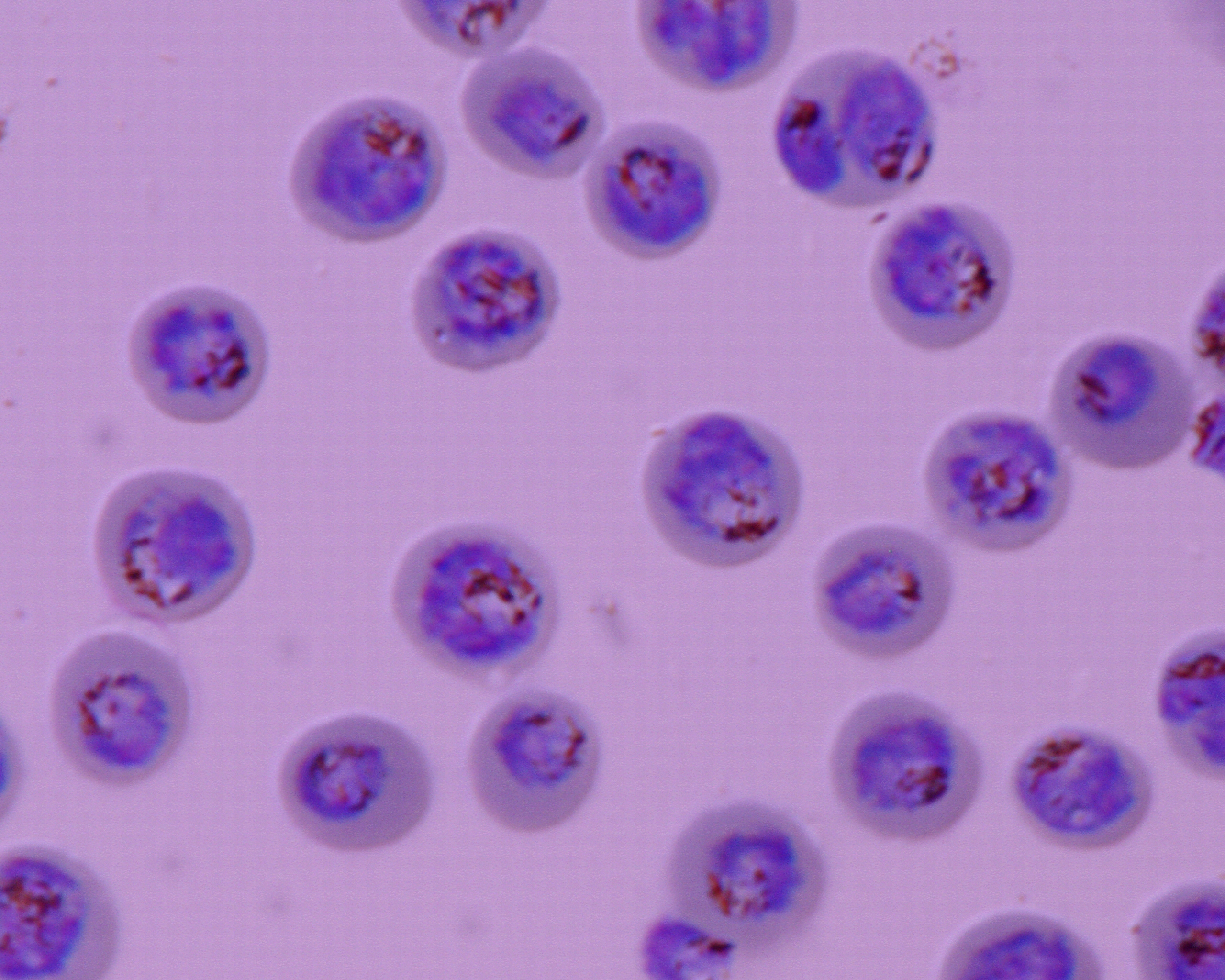
Our research team focuses on the identification and deciphering of the molecular interactions associated with the sequestration of erythrocytes infected by P. falciparum during placental malaria and cerebral malaria. Understanding the functional characteristics of parasite adhesion processes at the molecular level will provide a rational basis to accelerate the development of vaccines and therapies aimed at inhibiting the sequestration of infected erythrocytes causing severe forms of malaria.
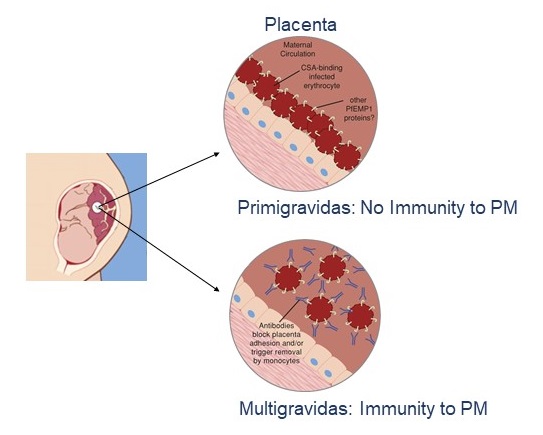
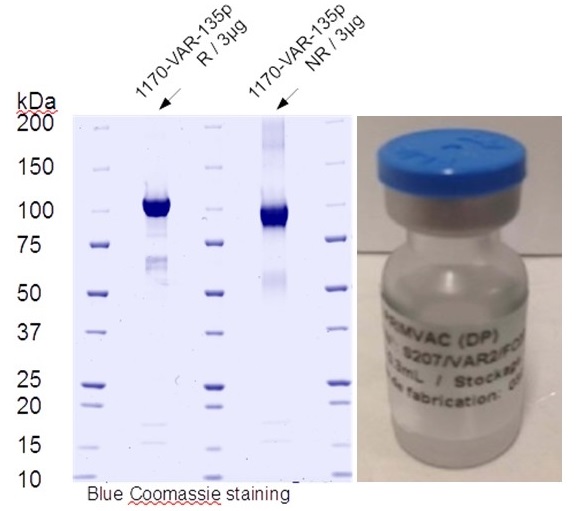
This strategy, which has been the DNA of our team since its creation, is at the origin of the development of the PRIMVAC vaccine aimed at preventing gestational malaria which was evaluated in a Phase I clinical trial and for which 3 other clinical trials including a phase II are programmed.



The team members
Our research focus on 3 axes
- Malaria
- Plasmodium
- Pregnancy
- Antibodies
- Vaccines
- Therapies
1. Host-parasite interactions associated to severe forms of malaria
In this axis we focus on the identification and the deciphering of the molecular interactions mediating infected erythocytes sequestration associated to placental malaria as well as cerebral malaria. These data will provide a rational basis for accelerating vaccine and therapeutic developments to inhibit IEs sequestration and therefore prevent pathogenesis and/or cure severe malaria. We are also notably assessing how the post-translational modifications of the parasite adhesins expressed on the surface of infected erythrocytes could modulate their trafficking to the host cell surface and cytoadhesion to the host receptors.
2. Vaccine development
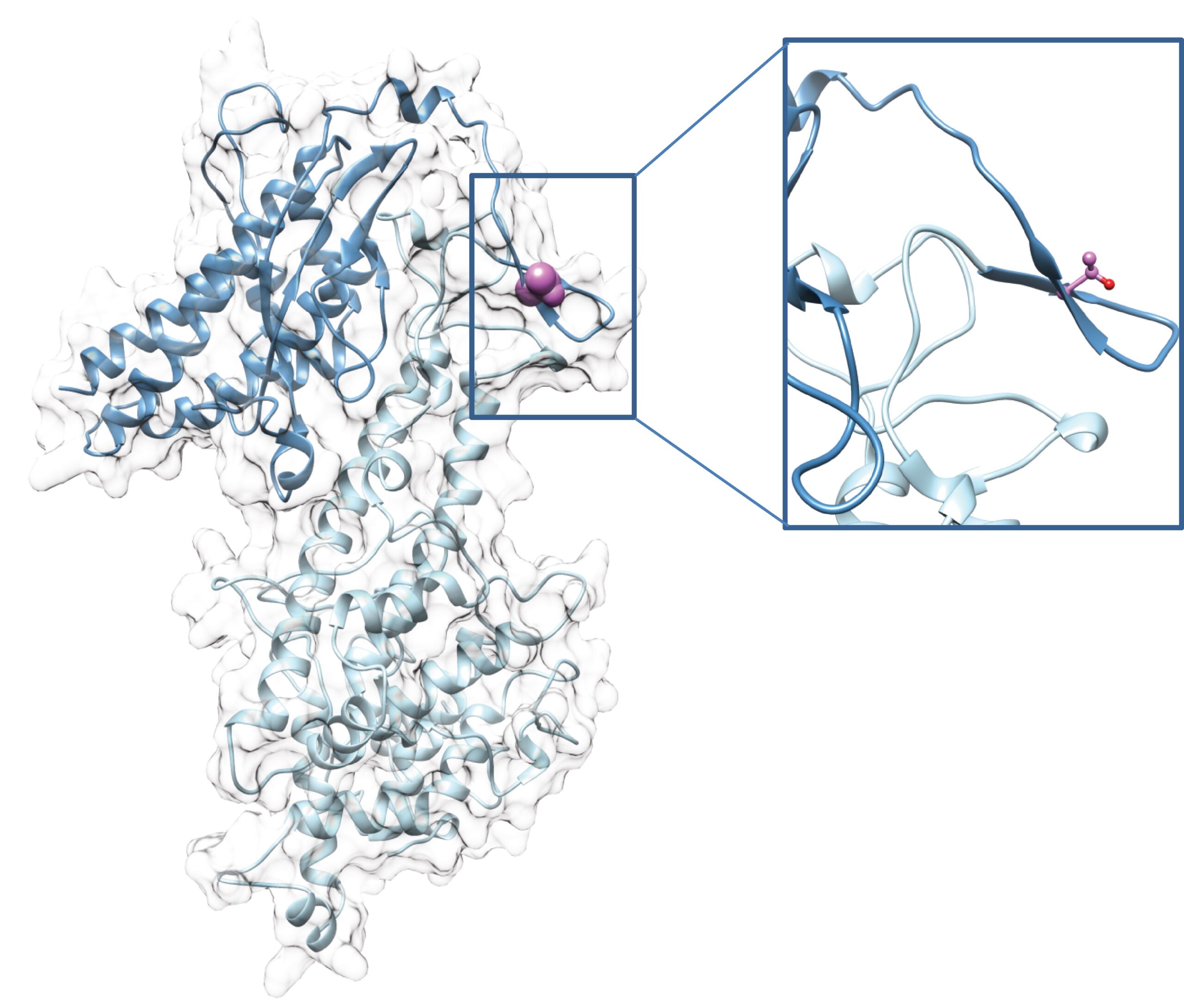
A vaccine that prevents P. falciparum malaria in pregnant women would save hundreds of thousands of lives each year. The results of the phase Ia/b clinical trials show that our placental malaria vaccine PRIMVAC was well tolerated and induced a strong immune response in all vaccinated women, with the production of antibodies that persisted for more than one year. We are currently characterizing the longevity of the PRIMVAC-induced immune response in women in Burkina Faso and the capacity of PRIMVAC to boost and broaden a natural acquired immune response in primigravid and multigravid women. Furthermore, in collaboration with European vaccine Initiative, we move forward PRIMVAC in a Phase II study in a clinical trial in Africa. We are also currently working on a second-generation vaccine that could broaden the immune response against the different VAR2CSA variants and on the development of a mRNA-based vaccine.
3. Develop new immunotherapeutic approaches
Despite a significant decrease in number of Plasmodium falciparum-related deaths in recent years, malaria remains a major public health problem. In this axis, we are developing Human monoclonal antibodies against VAR2CSA that could block binding of infected erythrocytes to the placenta. We are also developing and characterizing VHH/nanobodies against malaria antigens express at different life cycle stages. Nanobodies combine the advantages of binding activities of antibodies with properties of small molecules and can be engineered to add molecules. In that context we have developed a novel immunotherapeutic approach redirecting a pre-existing polyclonal antibody response against Epstein-Barr virus (EBV). We aim in generating bi-modular fusion proteins (BMFPs) able to recruit polyclonal endogenous high-affinity antibodies (anti-EBV) towards P. falciparum-infected erythrocytes. BMFPs will be designed based on an EBV antigen (P18) coupled to nanobody-derived binding moieties targeting P. falciparum (Pf) antigens specifically expressed at the surface of cells infected by asexual or sexual parasite forms.
The opportunities
- Cytoadhesion mechanisms
- Development of malaria vaccines
- Expression of recombinant proteins
- Development of monoclonal human antibodies and VHH
- New therapeutic strategies
Publications
Casein Kinases 2-dependent phosphorylation of the placental ligand VAR2CSA regulates Plasmodium falciparum-infected erythrocytes cytoadhesion. PLoS Pathog. 2025 Jan 13;21(1):e1012861.
JK–1, a useful erythroleukemic cell line model to study a controlled erythroid differentiation from progenitors to terminal erythropoiesis. Sci Rep. 2024 Oct 29;14(1):25885.
Sustained clinical benefit of malaria chemoprevention with sulfadoxine-pyrimethamine (SP) in pregnant women in a region with high SP resistance markers. J Infect. 2024 May;88(5):106144. doi: 10.1016/j.jinf.2024.106144. Epub 2024 Apr 2.
Duffy antigen is expressed during erythropoiesis in Duffy-negative individuals. Cell Host Microbe. 2023 Dec 13;31(12):2093-2106.e7. doi: 10.1016/j.chom.2023.10.019. Epub 2023 Dec 5.
Aotus nancymaae model predicts human immune response to the placental malaria vaccine candidate VAR2CSA. Lab Anim (NY). 2023 Dec;52(12):315-323. doi: 10.1038/s41684-023-01274-2. Epub 2023 Nov 6.
Detecting temporal and spatial malaria patterns from first antenatal care visits. Nat Commun. 2023 Jul 6;14(1):4004. doi: 10.1038/s41467-023-39662-4.
Malaria-specific Type 1 regulatory T cells are more abundant in first pregnancies and associated with placental malaria. EBioMedicine. 2023 Sep;95:104772. doi: 10.1016/j.ebiom.2023.104772. Epub 2023 Aug 25.
Associations between prenatal malaria exposure, maternal antibodies at birth, and malaria susceptibility during the first year of life in Burkina Faso. Infect Immun. 2023 Oct 17;91(10):e0026823. doi: 10.1128/iai.00268-23. Epub 2023 Sep 27.
An ACE2-Based Bimodular Fusion Protein Enables Reorientation of Endogenous Anti-Epstein-Barr Virus Antibodies Toward SARS-CoV-2 Spike. J Infect Dis. 2023 Dec 20;228(12):1675-1679. doi: 10.1093/infdis/jiad329.
Detecting temporal and spatial malaria patterns from first antenatal care visits. Res Sq [Preprint]. 2023 Feb 20:rs.3.rs-2592126. doi: 10.21203/rs.3.rs-2592126/v1.
Expression and Purification of scFv2H7-P18F3, a Bi-Modular Fusion Protein (BMFP) Targeting Human CD20. Bio Protoc. 2023 May 20;13(10):e4682. doi: 10.21769/BioProtoc.4682. eCollection 2023 May 20.
Evaluation of Malarial Var2CSA-Displaying Baculovirus Vector in Transduction Efficiency in Human Cancer Cells. Biol Pharm Bull. 2023;46(3):404-411. doi: 10.1248/bpb.b22-00630.